
Exhibit 99.1

CORPORATE PRESENTATION JULY 09, 2018 NASDAQ: APTO TSX: APS

2 This presentation does not, and is not intended to, constitute or form part of, and should not be construed as, an offer or invitation for the sale or purchase of, or a solicitation of an offer to purchase, subscribe for or otherwise acquire, any securities, businesses and/or assets of any entity, nor shall it or any part of it be relied upon in connection with or act as any inducement to enter into any contract or commitment or investment decision whatsoever . This presentation contains forward - looking statements, which reflect APTOSE Biosciences Inc . ’s (the “Company”) current expectations, estimates and projections regarding future events, including statements relating to our business strategy, our clinical development plans, our ability to obtain the substantial capital we require, our plans to secure strategic partnerships and to build our pipeline, our clinical trials and their projected timeline, the efficacy and toxicity of our product candidates, potential new intellectual property, our plans, objectives, expectations and intentions ; and other statements including words such as “anticipate”, “contemplate”, “continue”, “believe”, “plan”, “estimate”, “expect”, “intend”, “will”, “should”, “may”, and other similar expressions . Such statements constitute forward - looking statements within the meaning of securities laws . Although the Company believes that the views reflected in these forward - looking statements are reasonable, such statements involve significant risks and uncertainties, and undue reliance should not be placed on such statements . Certain material factors or assumptions are applied in making these forward - looking statements, and actual results may differ materially from those statements . Those factors and risks include, but are not limited to, our ability to raise the funds necessary to continue our operations, changing market conditions, the successful and timely completion of our clinical studies including delays, the demonstration of safety and efficacy of our drug candidates, our ability to recruit patients, the establishment and maintenance of corporate alliances, the market potential of our product candidates, the impact of competitive products and pricing, new product development, changes in laws and regulations, uncertainties related to the regulatory approval process and other risks detailed from time to time in the Company’s ongoing quarterly filings and annual reports . Forward - looking statements contained in this document represent views only as of the date hereof and are presented for the purpose of assisting potential investors in understanding the Company’s business, and may not be appropriate for other purposes . The Company does not undertake to update any forward - looking statements, whether written or oral, that may be made from time to time by or on its behalf, except as required under applicable securities legislation . Investors should read the Company’s continuous disclosure documents available at www . sedar . com and EDGAR at www . sec . gov/edgar . shtml , especially the risk factors detailed therein .

3 Aptose Corporate Snapshot Clinical stage company employing a mechanism - driven approach to deliver safer, targeted, first - in - class cancer drugs Public Company NASDAQ: APTO / TSX: APS Shares Outs. (6/30/2018) Basic: 34.4 MM; FD: 38.8 MM No Warrants / No Preferred Stock / No Debt 3 Month ADTV ~400,000 Shares Market Cap (6/30/2018) ~$130 Million Cash Position (3/31/18) US$16.2 Million / CA$20.9 Million Cash Runway >12 Months Executive Headquarters & Research Laboratories San Diego, CA

4 Aptose Investment Highlights Clinical stage biotechnology company developing first - in - class targeted agents to treat life - threatening hematological malignancies / orphan opportunities Two differentiated targeted agents with Strong IP Protection APTO - 253 : MYC Inhibitor - Only clinical stage agent directly targeting MYC oncogene - Currently at Phase Ib stage for acute myeloid leukemia (AML) CG - 806 : Oral Pan - FLT3 / Pan - BTK Inhibitor - Potent inhibitor of wild type & all mutant FLT3 >> AML - Potent inhibitor of wild type & all mutant BTK >> B - cell Cancers - IND planned 2018 to support AML and B - cell cancer FIH trials $1B+ commercial opportunity in lead indications (AML and CLL) Strong leadership team of industry, financial and clinical research professionals FDA Orphan Drug Designation in AML FDA Orphan Drug Designation in AML

5 Aptose Leadership Team – See www.aptose.com Dr. William G. Rice, PhD Chairman, President & CEO Achillion Pharmaceuticals: Founder, CEO, President, CSO, Director National Cancer Institute - FCRDC: Sr. Scientist, Drug Mechanism Lab Cylene Pharmaceuticals: Chairman, CEO, President, CSO Mr. Gregory Chow Sr. Vice President & CFO RBC Capital Markets: Director, Led Life Sciences Private Placements Wells Fargo: Led Private Capital Group BDO Seidman, LLP: Senior Auditor, CPA (inactive), State of California Dr. Hannah Zhang, MD, PhD Sr. Director of Research Aangstrom Pharm: Project Mgr to Moores Cancer Center Bio - Quant: Sr. Research Scientist Guilin Medical College, Guilin, P.R. China: Ob.Gyn. Dr. Stephen Howell, MD Serves as Chief Medical Officer Distinguished Professor of Medicine, UCSD Moore’s Cancer Center Physician scientist conducting research to address drug resistance Expertise in pharmacology and design and conduct of clinical trials Mr. Ernest Kitt VP, Dev’t & Technical Operations Amgen/Onyx: Molecule Lead Director for Kyprolis in Clinical Operations Oncosec Medical: Executive Director of Clinical Operations Medicinova Inc: Associate Director of Clinical Operations

6 Aptose Scientific/Clinical Advisory Team Dr. Brian J. Druker, MD Collaborator & Chair of SAB Key Role in Dev’t of Gleevec Member, National Academy of Sciences Winner of Karnofsky Award and Lasker “ America ’ s Nobel ” Award Leader of Inter - institutional Beat AML Initiative Dr. Michael Andreeff, MD, PhD Collaborator & Member of SAB Professor of Medicine, Chair in Genetics, MD Anderson Cancer Center Physician Scientist, expert in AML / drug resistance / drug mechanisms, published over 450 peer - reviewed papers / books / chapters Dr. Daniel Von Hoff, MD, FACP Serves as SVP of Medical Affairs Winner of 2010 Karnofsky Memorial Award Prior President of AACR Board Member of ASCO Appointed to President’s National Cancer Advisory Board Scientific Advisory Board Populated with KOLs – Domain Expertise
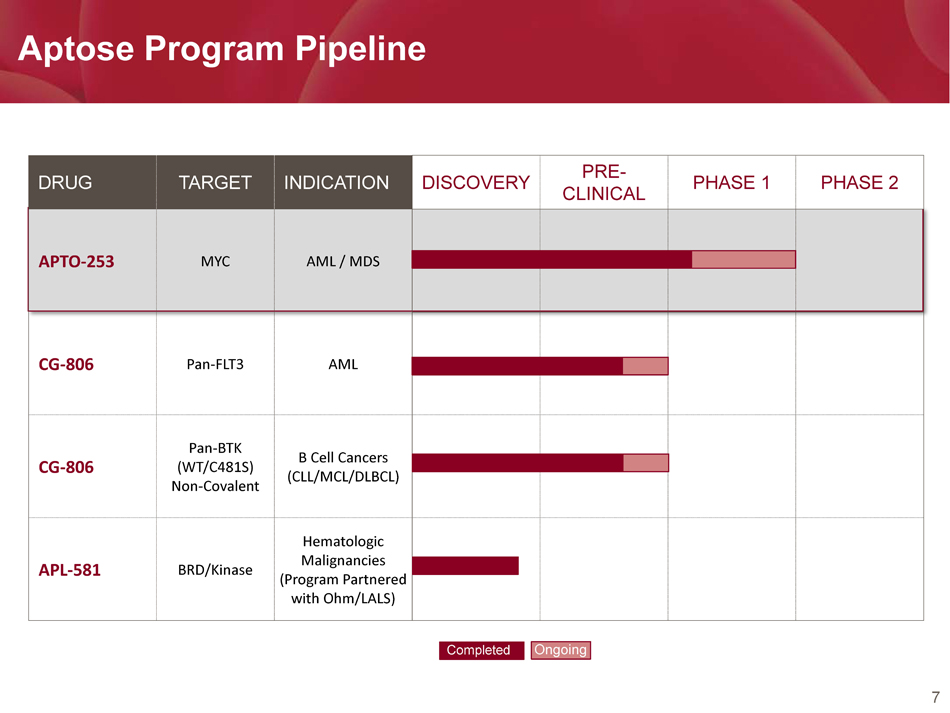
7 Aptose Program Pipeline DRUG TARGET INDICATION DISCOVERY PRE - CLINICAL PHASE 1 PHASE 2 APTO - 253 MYC AML / MDS CG - 806 Pan - FLT3 AML CG - 806 Pan - BTK (WT/C481S) Non - Covalent B Cell Cancers (CLL/MCL/DLBCL) APL - 581 BRD/Kinase Hematologic Malignancies (Program Partnered with Ohm/LALS) Completed Ongoing

APTO - 253 Phase 1b Stage First - in - Class Inhibitor of MYC Expression
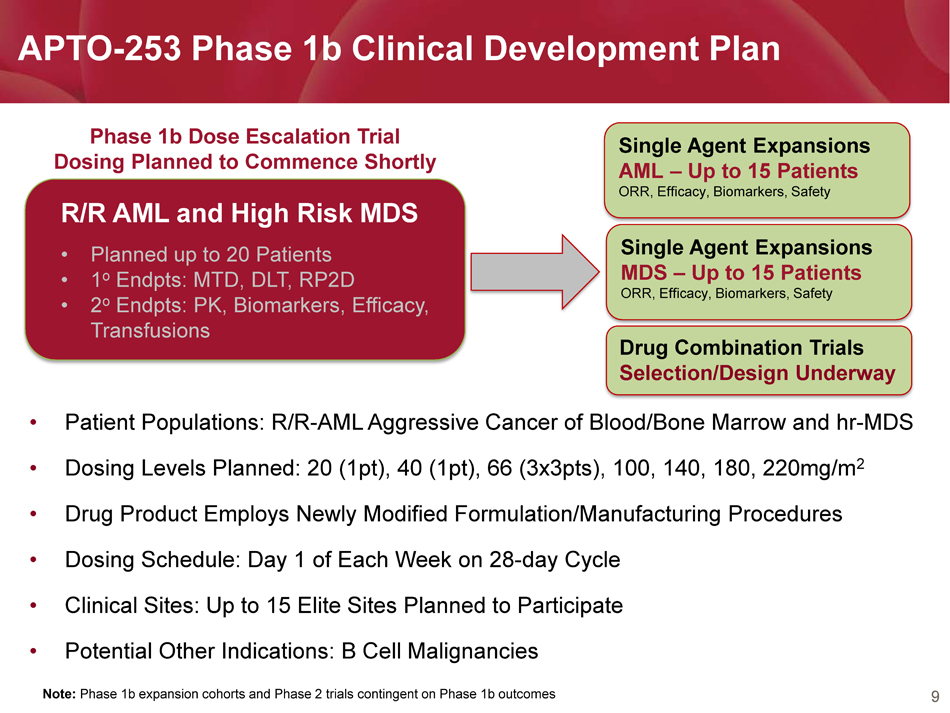
9 APTO - 253 Phase 1b Clinical Development Plan Note: Phase 1b expansion cohorts and Phase 2 trials contingent on Phase 1b outcomes R/R AML and High Risk MDS • Planned up to 20 Patients • 1 o Endpts: MTD, DLT, RP2D • 2 o Endpts: PK, Biomarkers, Efficacy, Transfusions Single Agent Expansions MDS – Up to 15 Patients ORR, Efficacy, Biomarkers, Safety Phase 1b Dose Escalation Trial Dosing Planned to Commence Shortly Single Agent Expansions AML – Up to 15 Patients ORR, Efficacy, Biomarkers, Safety • Patient Populations: R/R - AML Aggressive Cancer of Blood/Bone Marrow and hr - MDS • Dosing Levels Planned: 20 (1pt), 40 (1pt), 66 (3x3pts), 100, 140, 180, 220mg/m 2 • Drug Product Employs Newly Modified Formulation/Manufacturing Procedures • Dosing Schedule: Day 1 of Each Week on 28 - day Cycle • Clinical Sites: Up to 15 Elite Sites Planned to Participate • Potential Other Indications: B Cell Malignancies Drug Combination Trials Selection/Design Underway
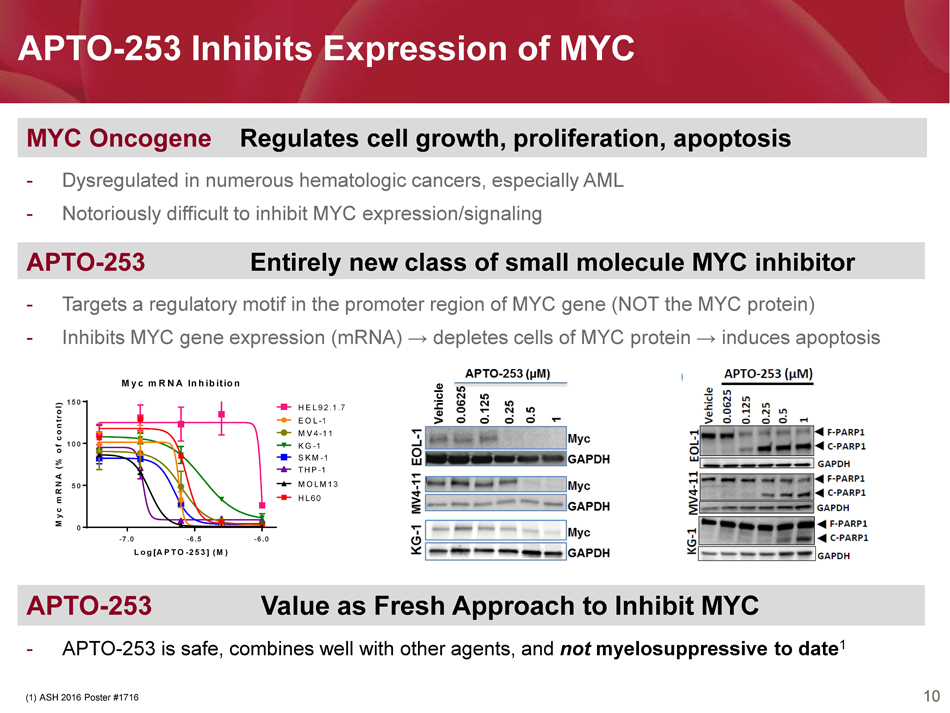
10 APTO - 253 Inhibits Expression of MYC MYC Oncogene Regulates cell growth, proliferation, apoptosis - Dysregulated in numerous hematologic cancers , especially AML - Notoriously difficult to inhibit MYC e xpression/signaling APTO - 253 Value as Fresh Approach to Inhibit MYC - APTO - 253 is safe, combines well with other agents, and not myelosuppressive to date 1 APTO - 253 Entirely new class of small molecule MYC inhibitor - Targets a regulatory motif in the promoter region of MYC gene (NOT the MYC protein) - Inhibits MYC gene expression (mRNA) → depletes cells of MYC protein → induces apoptosis (1) ASH 2016 Poster #1716 -7.0 -6.5 -6.0 0 50 100 150 Myc mRNA Inhibition Log[APTO-253] (M) M y c m R N A ( % o f c o n t r o l ) EOL-1 HEL92.1.7 KG-1 MV4-11 SKM-1 THP-1 MOLM13 HL60

11 APTO - 253 stabilizes G - quadruplex DNA, inhibits MYC expression and induces DNA damage in acute myeloid leukemia cells Andrea Local, Hongying Zhang, Khalid D Benbatoul, Peter Folger, Xia Sheng, Cheng - Yu Tsai, Stephen B. Howell, and William G. Rice AACR Journal: Molecular Cancer Therapeutics , June 2018 (Volume 17, Number 6) Binding/Stabilizing G - quadruplex DNA (G4) motif in MYC promoter silences MYC gene expression APTO - 253 is a new addition to the repertoire of drugs that can exploit DNA BRCA1/2 deficiency Cheng - Yu Tsai, Si Sun, Hongying Zhang, Andrea Local, Yongxuan Su, Larry A Gross, William Rice, and Stephen B. Howell AACR Journal: Molecular Cancer Therapeutics , June 2018 (Volume 17, Number 6) Binding of G4 can destabilize telomeres and stall replication forks resulting in DNA damage response Cancer cells harboring BRCA1/2 mutations hypersensitive – new solid tumor path APTO - 253 is a new addition to the repertoire of drugs that can exploit DNA BRCA1/2 deficiency Cheng - Yu Tsai, Si Sun, Hongying Zhang, Andrea Local, William Rice, and Stephen B. Howell 2018 AACR Abstract and Poster Presentation APTO - 253 Mechanism & Therapeutic Indications: AACR Publications and 2018 AACR Presentation
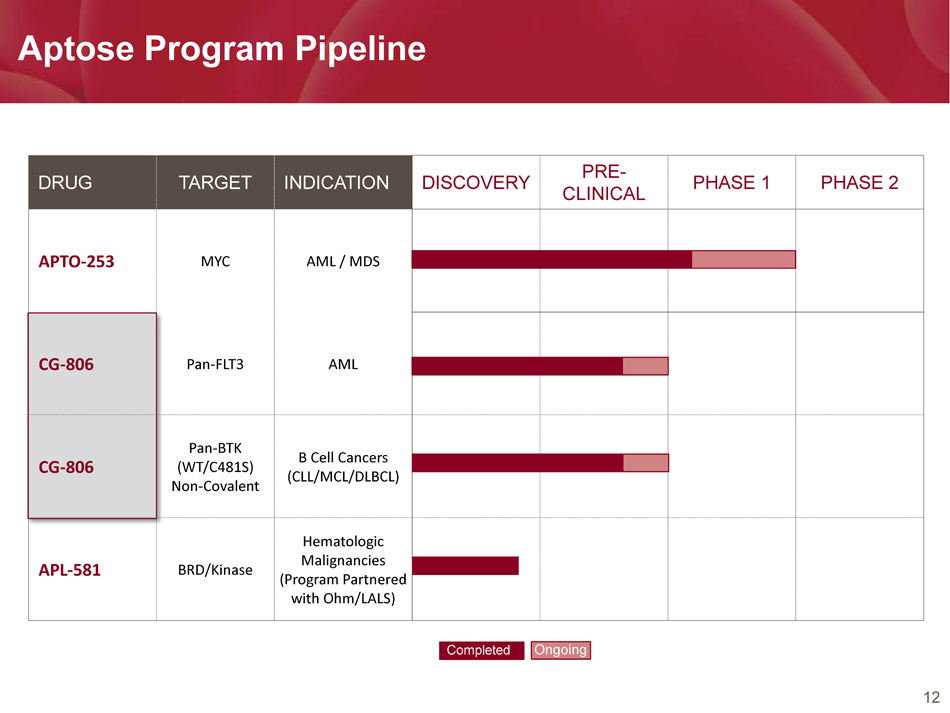
12 Aptose Program Pipeline DRUG TARGET INDICATION DISCOVERY PRE - CLINICAL PHASE 1 PHASE 2 APTO - 253 MYC AML / MDS CG - 806 Pan - FLT3 AML CG - 806 Pan - BTK (WT/C481S) Non - Covalent B Cell Cancers (CLL/MCL/DLBCL) APL - 581 BRD/Kinase Hematologic Malignancies (Program Partnered with Ohm/LALS) Completed Ongoing

CG - 806 First - in - Class Pan - FLT3 / Pan - BTK “Multi - Cluster” Kinase Inhibitor
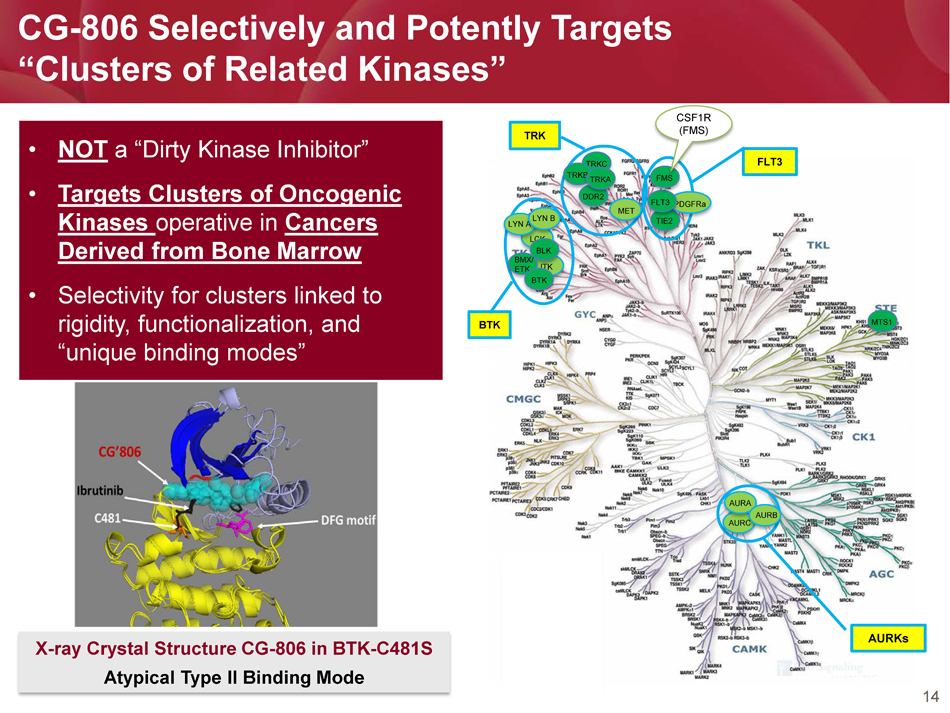
• NOT a “Dirty Kinase Inhibitor” • Targets Clusters of Oncogenic Kinases operative in Cancers Derived from Bone Marrow • Selectivity for clusters linked to rigidity, functionalization, and “unique binding modes” 14 CG - 806 Selectively and Potently Targets “Clusters of Related Kinases” PDGFR TRKB AURKs BTK FLT3 TRK LCK ITK LYN A LYN B BLK BMX/ ETK BTK TRKC DDR2 TRKB TIE2 TRKA MET PDGFRa FLT3 MTS1 AURC AURA AURB FMS CSF1R (FMS) X - ray Crystal Structure CG - 806 in BTK - C481S Atypical Type II Binding Mode
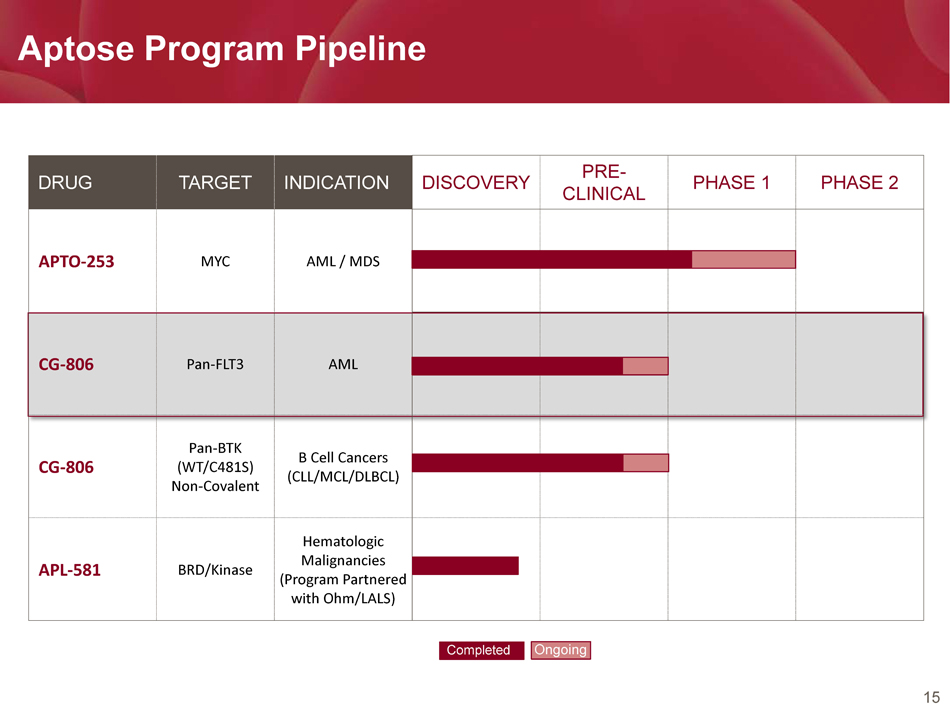
15 Aptose Program Pipeline DRUG TARGET INDICATION DISCOVERY PRE - CLINICAL PHASE 1 PHASE 2 APTO - 253 MYC AML / MDS CG - 806 Pan - FLT3 AML CG - 806 Pan - BTK (WT/C481S) Non - Covalent B Cell Cancers (CLL/MCL/DLBCL) APL - 581 BRD/Kinase Hematologic Malignancies (Program Partnered with Ohm/LALS) Completed Ongoing
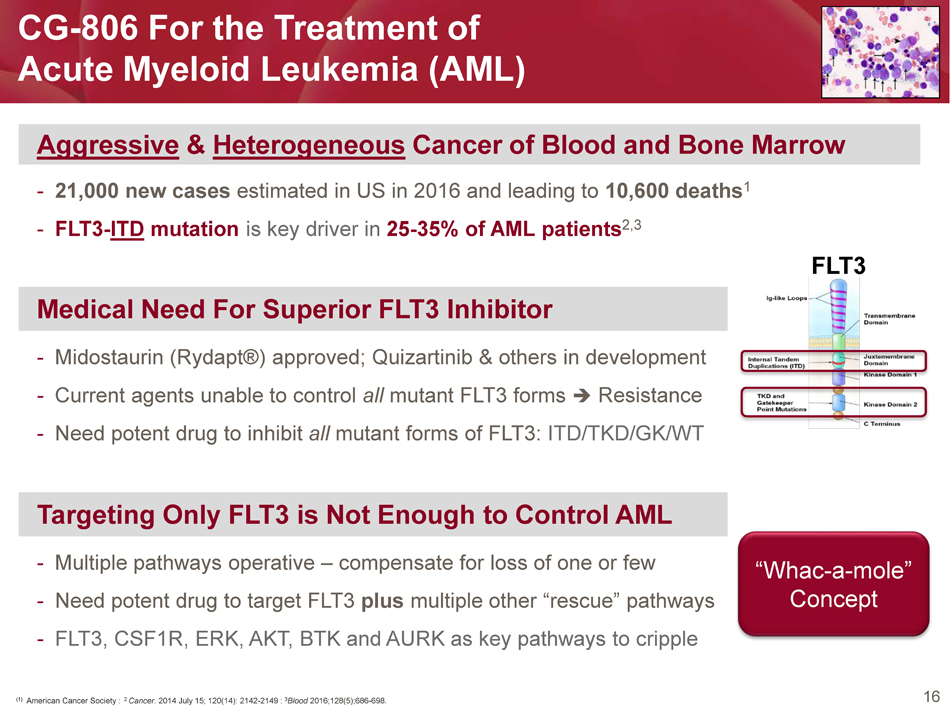
16 CG - 806 For the Treatment of Acute Myeloid Leukemia (AML) Aggressive & Heterogeneous Cancer of Blood and Bone Marrow - 21,000 new cases estimated in US in 2016 and leading to 10,600 deaths 1 - FLT3 - ITD mutation is key driver in 25 - 35% of AML patients 2,3 (1) American Cancer Society : 2 Cancer . 2014 July 15; 120(14): 2142 - 2149 : 3 Blood 2016;128(5);686 - 698. FLT3 Medical Need For Superior FLT3 Inhibitor - Midostaurin ( Rydapt ®) approved; Quizartinib & others in development - Current agents unable to control all mutant FLT3 forms Resistance - Need potent drug to inhibit all mutant forms of FLT3: ITD/TKD/GK/WT Targeting Only FLT3 is Not Enough to Control AML - Multiple pathways operative – compensate for loss of one or few - Need potent drug to target FLT3 plus multiple other “rescue” pathways - FLT3, CSF1R, ERK, AKT, BTK and AURK as key pathways to cripple “ Whac - a - mole” Concept
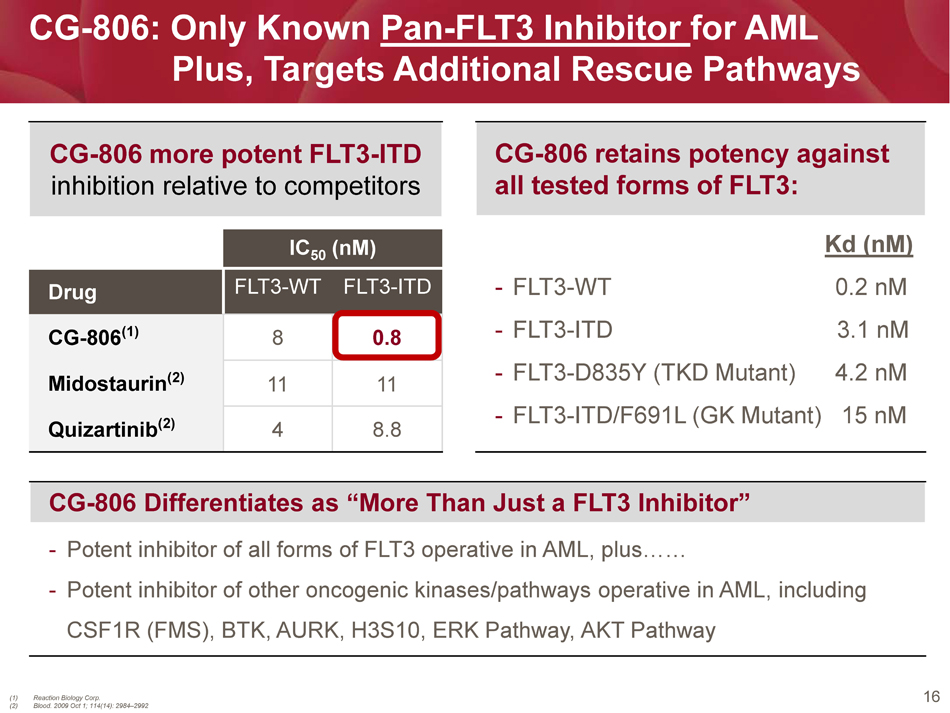
16 CG - 806: Only Known Pan - FLT3 Inhibitor for AML Plus, Targets Additional Rescue Pathways IC 50 ( nM ) Drug FLT3 - WT FLT3 - ITD CG - 806 (1) 8 0.8 Midostaurin (2) 11 11 Quizartinib (2) 4 8.8 (1) Reaction Biology Corp. (2) Blood. 2009 Oct 1; 114(14): 2984 – 2992 CG - 806 more potent FLT3 - ITD inhibition relative to competitors CG - 806 Differentiates as “More Than Just a FLT3 Inhibitor” - Potent inhibitor of all forms of FLT3 operative in AML, plus…… - Potent inhibitor of other oncogenic kinases/pathways operative in AML, including CSF1R (FMS), BTK, AURK, H3S10, ERK Pathway, AKT Pathway CG - 806 retains potency against all tested forms of FLT3: Kd ( nM ) - FLT3 - WT 0.2 nM - FLT3 - ITD 3.1 nM - FLT3 - D835Y (TKD Mutant) 4.2 nM - FLT3 - ITD/ F691L (GK Mutant) 15 nM
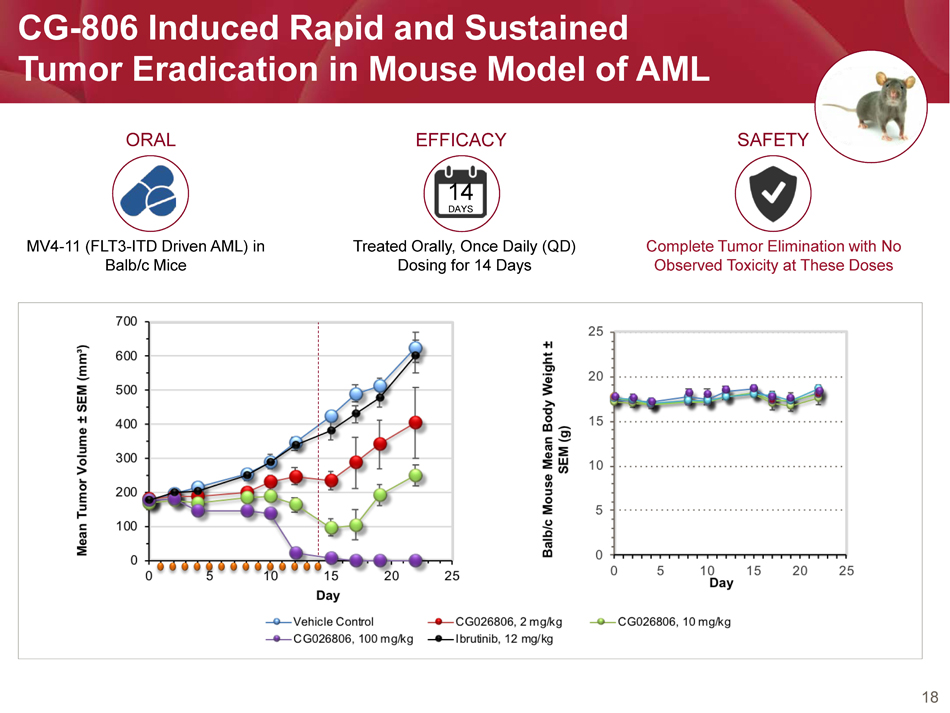
18 CG - 806 Induced Rapid and Sustained Tumor Eradication in Mouse Model of AML ORAL MV4 - 11 (FLT3 - ITD Driven AML) in Balb/c Mice EFFICACY 14 DAYS Treated Orally, Once Daily (QD) Dosing for 14 Days SAFETY Complete Tumor Elimination with No Observed Toxicity at These Doses
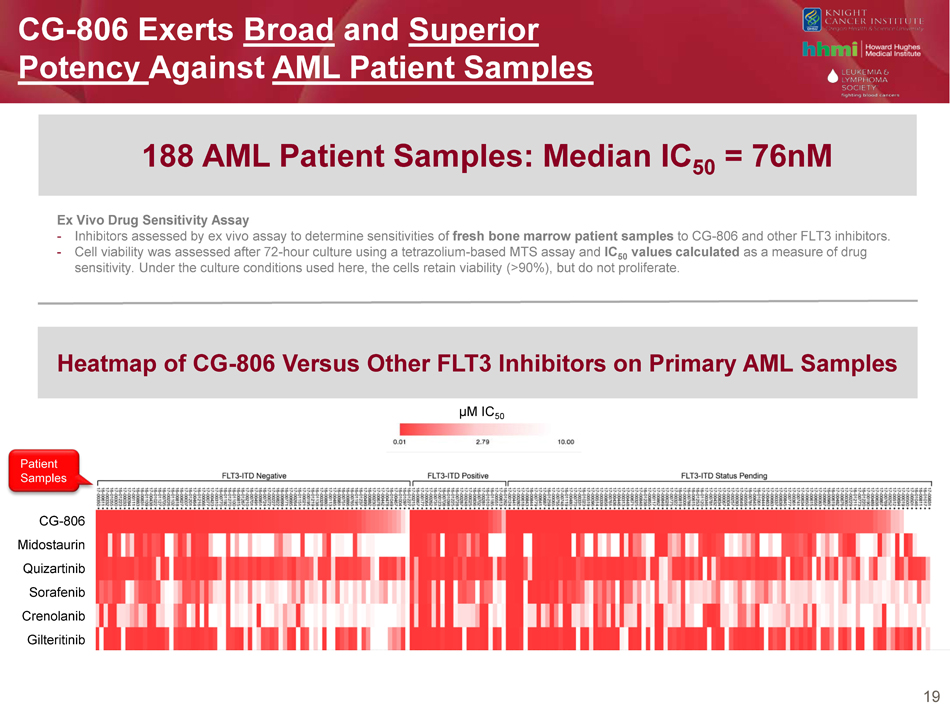
19 CG - 806 Exerts Broad and Superior Potency Against AML Patient Samples CG - 806 Midostaurin Quizartinib Sorafenib Crenolanib Gilteritinib Patient Samples Heatmap of CG - 806 Versus Other FLT3 Inhibitors on Primary AML Samples 188 AML Patient Samples: Median IC 50 = 76nM Ex Vivo Drug Sensitivity Assay - Inhibitors assessed by ex vivo assay to determine sensitivities of fresh bone marrow patient samples to CG - 806 and other FLT3 inhibitors. - Cell viability was assessed after 72 - hour culture using a tetrazolium - based MTS assay and IC 50 values calculated as a measure of drug sensitivity. Under the culture conditions used here, the cells retain viability (>90%), but do not proliferate. µM IC 50
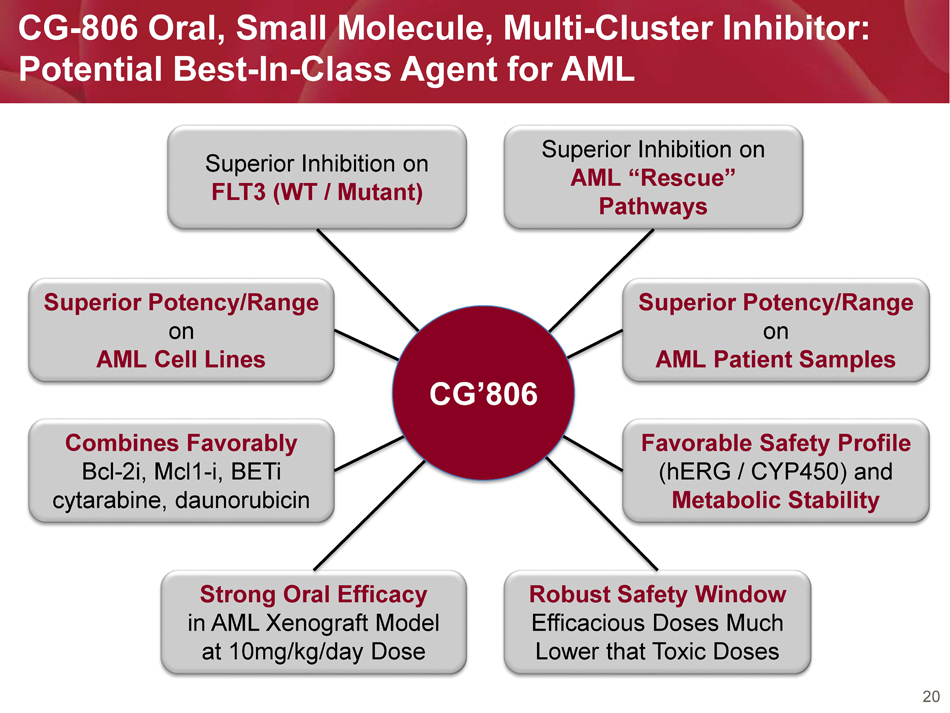
20 CG - 806 Oral, Small Molecule, Multi - Cluster Inhibitor: Potential Best - In - Class Agent for AML Superior Inhibition on FLT3 (WT / Mutant) Superior Inhibition on AML “Rescue” Pathways Superior Potency/Range on AML Cell Lines Superior Potency/Range on AML Patient Samples Combines Favorably Bcl - 2i, Mcl1 - i, BETi cytarabine, daunorubicin Favorable Safety Profile (hERG / CYP450) and Metabolic Stability Strong Oral Efficacy in AML Xenograft Model at 10mg/kg/day Dose Robust Safety Window Efficacious Doses Much Lower that Toxic Doses CG’806
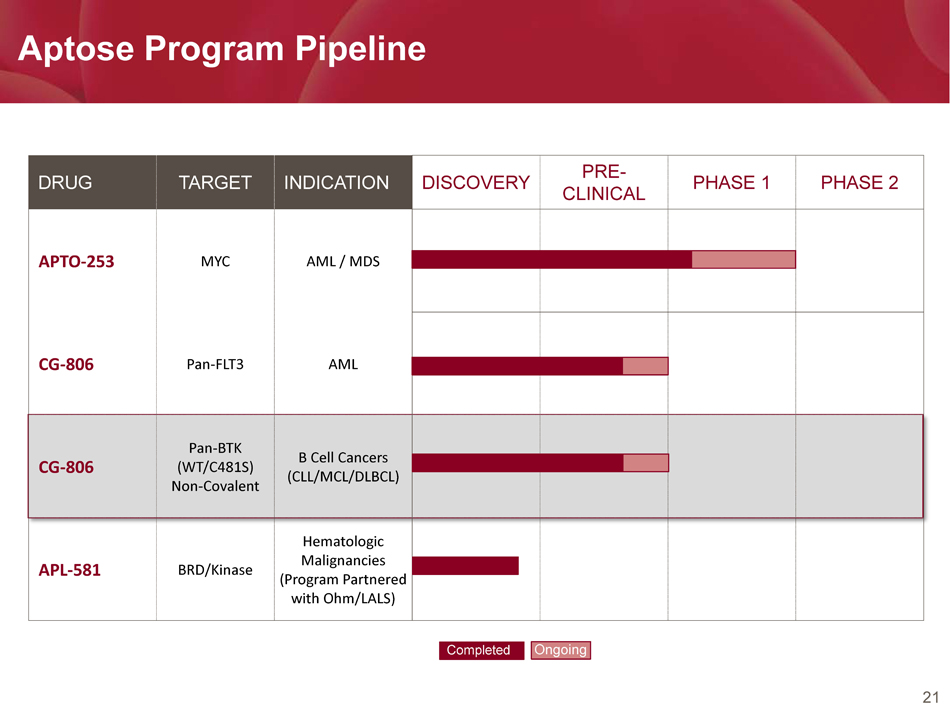
21 Aptose Program Pipeline DRUG TARGET INDICATION DISCOVERY PRE - CLINICAL PHASE 1 PHASE 2 APTO - 253 MYC AML / MDS CG - 806 Pan - FLT3 AML CG - 806 Pan - BTK (WT/C481S) Non - Covalent B Cell Cancers (CLL/MCL/DLBCL) APL - 581 BRD/Kinase Hematologic Malignancies (Program Partnered with Ohm/LALS) Completed Ongoing
22 Medical Need for Next Generation BTK Inhibitor Ibrutinib (Imbruvica ® ) is Current Standard of Care - $Multi - billion WW sales in 2017 (Bloomberg) Ibrutinib Shortcomings – Patients Discontinuing - 51% CLL Patients: D iscontinue treatment with ibrutinib after 3.4yrs (1) - 5 - 10% P atients: Resistant (C481S) to ibrutinib C ovalent inhibitor - 40 - 45% Patients: Intolerant or refractory to ibrutinib Overexpressed BTK Drives Signaling in B Cell Malignancies - CLL, MCL, DLBCL (1) Woyach et al. J Clin Oncol ..; 2017: 35; 1 - 7 CG - 806 Overcomes Shortcomings of Ibrutinib • “Non - covalent inhibitor” of BTK (WT & C481S) • Well tolerated in animal toxicology studies • Inhibits multiple “rescue” kinases/pathways • Plan to treat all patients discontinuing ibrutinib
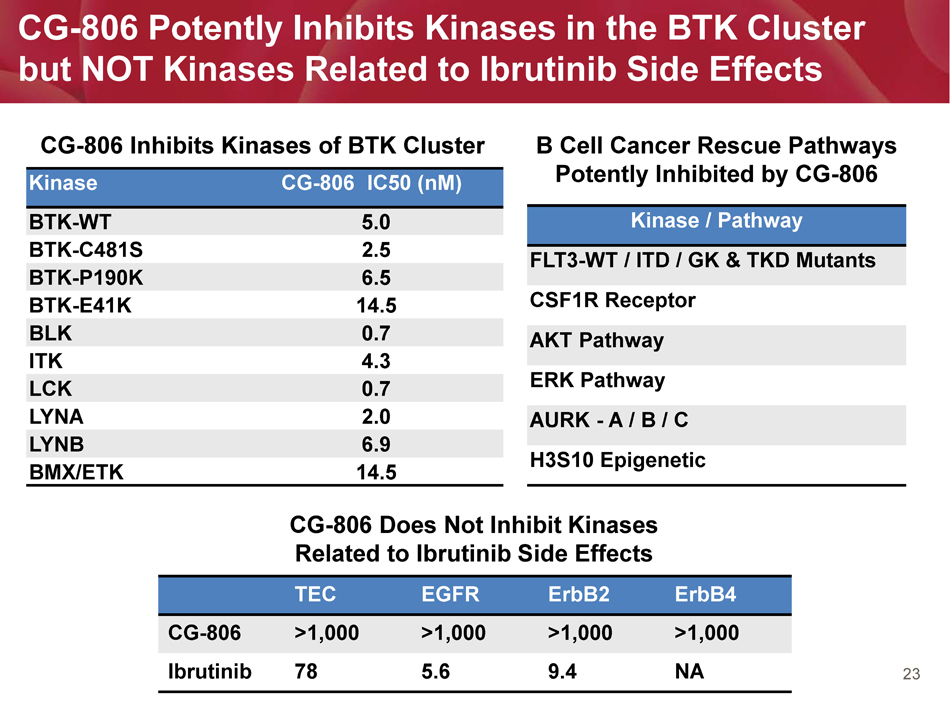
23 Kinase CG - 806 IC50 ( nM ) BTK - WT 5.0 BTK - C481S 2.5 BTK - P190K 6.5 BTK - E41K 14.5 BLK 0.7 ITK 4.3 LCK 0.7 LYNA 2.0 LYNB 6.9 BMX/ETK 14.5 CG - 806 Potently Inhibits Kinases in the BTK Cluster but NOT Kinases Related to Ibrutinib Side Effects TEC EGFR ErbB2 ErbB4 CG - 806 >1,000 >1,000 >1,000 >1,000 Ibrutinib 78 5.6 9.4 NA CG - 806 Does Not Inhibit Kinases Related to Ibrutinib Side Effects CG - 806 Inhibits Kinases of BTK Cluster Kinase / Pathway FLT3 - WT / ITD / GK & TKD Mutants CSF1R Receptor AKT Pathway ERK Pathway AURK - A / B / C H3S10 Epigenetic B Cell Cancer Rescue Pathways Potently Inhibited by CG - 806
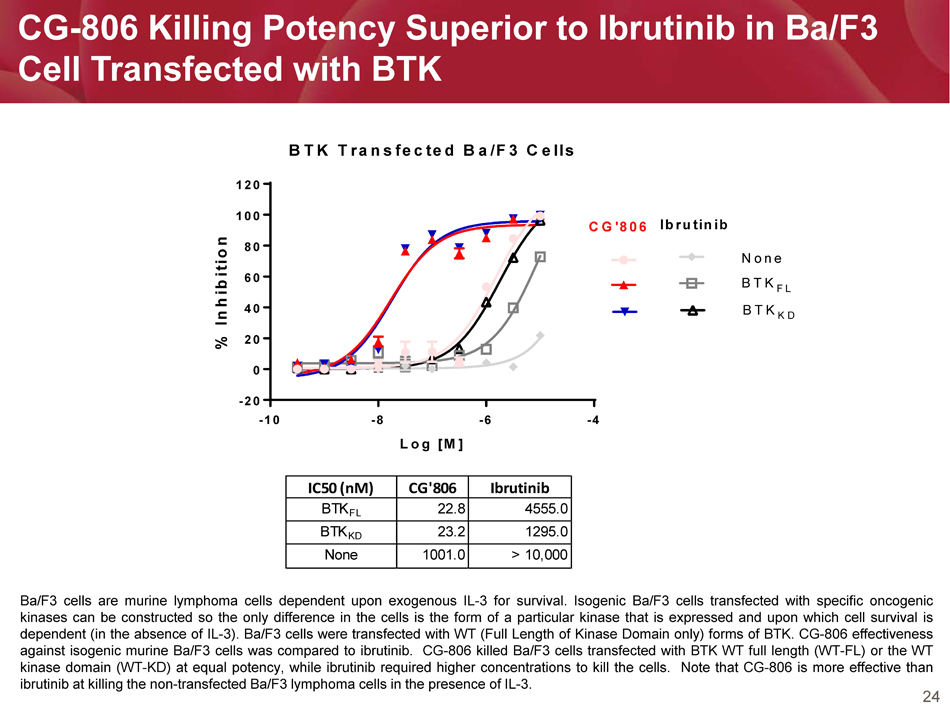
24 CG - 806 Killing Potency Superior to Ibrutinib in Ba/F3 Cell Transfected with BTK -10 -8 -6 -4 -20 0 20 40 60 80 100 120 BTK Transfected Ba/F3 Cells Log [M] % I n h i b i t i o n None BTK FL BTK KD CG'806 Ibrutinib IC50 (nM) CG'806 Ibrutinib BTK FL 22.8 4555.0 BTK KD 23.2 1295.0 None 1001.0 > 10,000 Ba/F 3 cells are murine lymphoma cells dependent upon exogenous IL - 3 for survival . Isogenic Ba/F 3 cells transfected with specific oncogenic kinases can be constructed so the only difference in the cells is the form of a particular kinase that is expressed and upon which cell survival is dependent (in the absence of IL - 3 ) . Ba/F 3 cells were transfected with WT (Full Length of Kinase Domain only) forms of BTK . CG - 806 effectiveness against isogenic murine Ba/F 3 cells was compared to ibrutinib . CG - 806 killed Ba/F 3 cells transfected with BTK WT full length (WT - FL) or the WT kinase domain (WT - KD) at equal potency, while ibrutinib required higher concentrations to kill the cells . Note that CG - 806 is more effective than ibrutinib at killing the non - transfected Ba/F 3 lymphoma cells in the presence of IL - 3 .
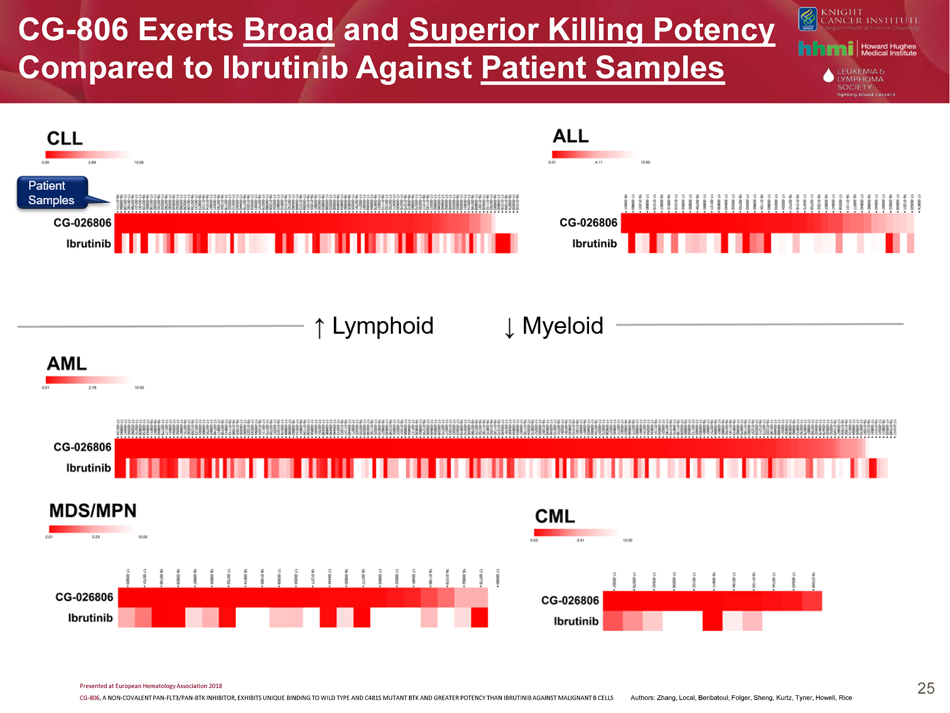
CG - 806 Exerts Broad and Superior Killing Potency Compared to Ibrutinib Against Patient Samples 25 Presented at European Hematology Association 2018 CG - 806 , A NON - COVALENT PAN - FLT3/PAN - BTK INHIBITOR, EXHIBITS UNIQUE BINDING TO WILD TYPE AND C481S MUTANT BTK AND GREATER POTENCY THAN IBRUTINIB AGAINST MALIGNANT B CELLS Authors: Zhang, Local, Benbatoul, Folger, Sheng, Kurtz, Tyner, Howell, Rice
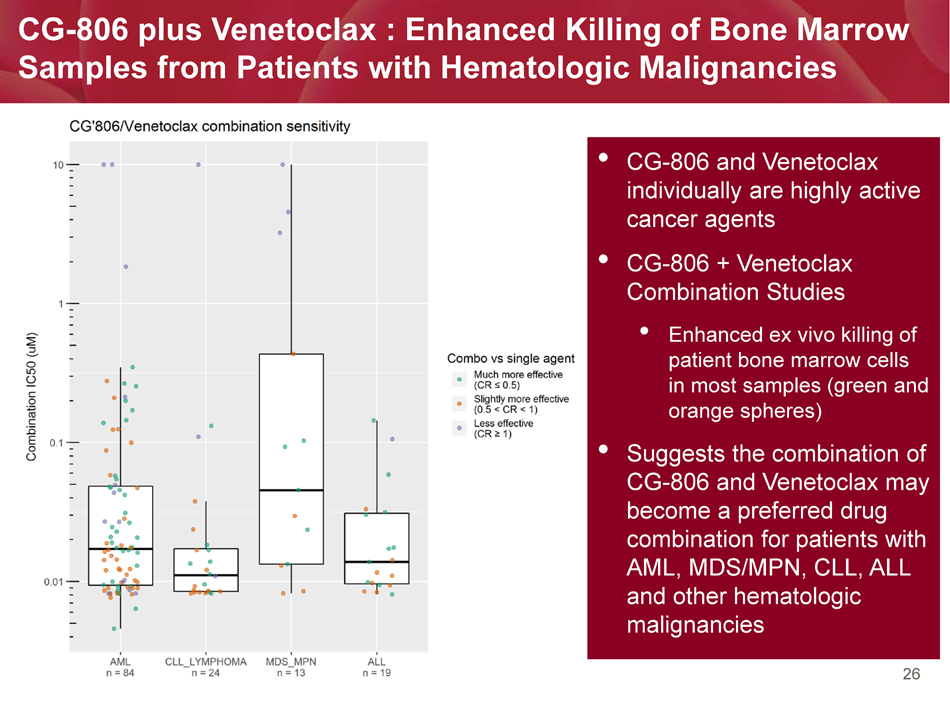
26 • CG - 806 and Venetoclax individually are highly active cancer agents • CG - 806 + Venetoclax Combination Studies • Enhanced ex vivo killing of patient bone marrow cells in most samples (green and orange spheres) • Suggests the combination of CG - 806 and Venetoclax may become a preferred drug combination for patients with AML, MDS/MPN, CLL, ALL and other hematologic malignancies CG - 806 plus Venetoclax : Enhanced Killing of Bone Marrow Samples from Patients with Hematologic Malignancies
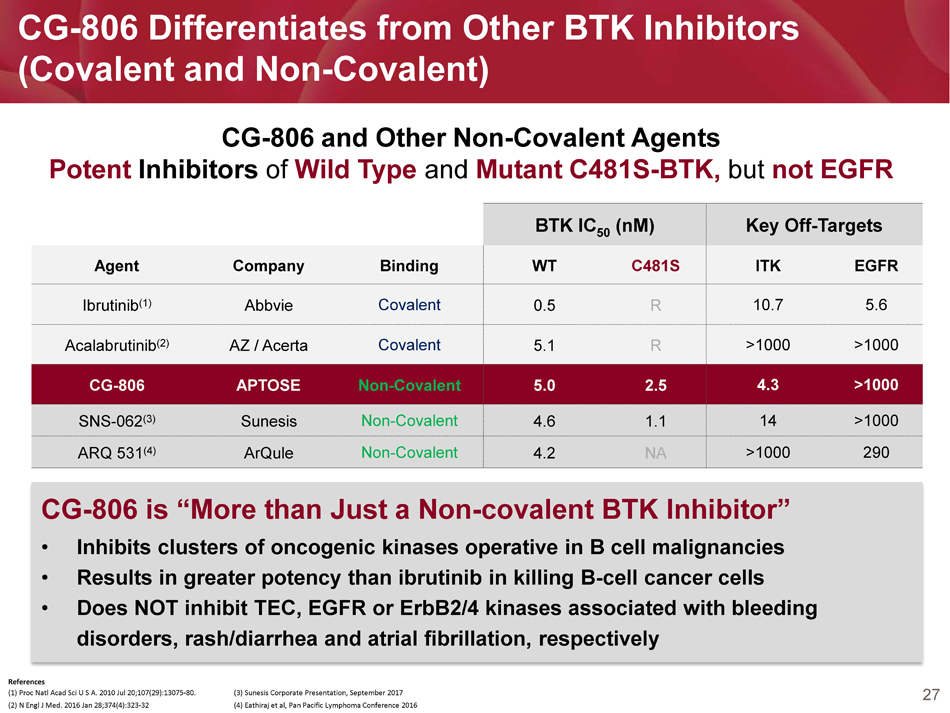
27 CG - 806 Differentiates from Other BTK Inhibitors (Covalent and Non - Covalent) BTK IC 50 (nM) Key Off - Targets Agent Company Binding WT C481S ITK EGFR Ibrutinib (1) Abbvie Covalent 0.5 R 10.7 5.6 Acalabrutinib (2) AZ / Acerta Covalent 5.1 R >1000 >1000 CG - 806 APTOSE Non - Covalent 5.0 2.5 4.3 >1000 SNS - 062 (3) Sunesis Non - Covalent 4.6 1.1 14 >1000 ARQ 531 (4) ArQule Non - Covalent 4.2 NA >1000 290 CG - 806 and Other Non - Covalent Agents Potent Inhibitors of Wild Type and Mutant C481S - BTK, but not EGFR References (1) Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13075 - 80. (3) Sunesis Corporate Presentation, September 2017 (2) N Engl J Med. 2016 Jan 28;374(4):323 - 32 (4) Eathiraj et al, Pan Pacific Lymphoma Conference 2016 CG - 806 is “More than Just a Non - covalent BTK Inhibitor” • Inhibits clusters of oncogenic kinases operative in B cell malignancies • Results in greater potency than ibrutinib in killing B - cell cancer cells • Does NOT inhibit TEC, EGFR or ErbB2/4 kinases associated with bleeding disorders, rash/diarrhea and atrial fibrillation, respectively
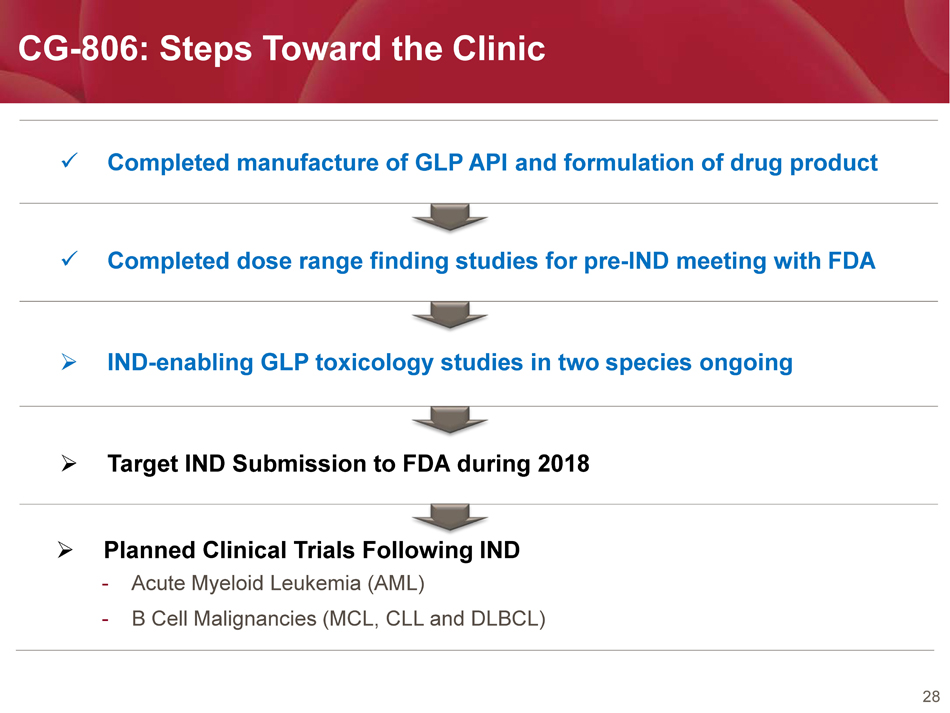
28 CG - 806: Steps Toward the Clinic x Completed manufacture of GLP API and formulation of drug product x Completed d ose range finding studies for pre - IND meeting with FDA » IND - enabling GLP toxicology studies in two species ongoing » Target IND Submission to FDA during 2018 » Planned Clinical Trials Following IND - Acute Myeloid Leukemia ( AML ) - B Cell Malignancies ( MCL, CLL and DLBCL )

29 Aptose Executive Summary Developing Highly Differentiated / Targeted Drugs for Blood Cancers APTO - 253 First - in - Class MYC Expression Inhibitor in Phase Ib for AML - Expect to commence screening and dosing of R/R - AML / hr - MDS patients soon - Potential to expand into B cell malignancy patients (role of MYC) - New modified formulation creates potential for new IP CG - 806 First - in - Class Pan - FLT3 / Pan - BTK Multi - Cluster Inhibitor - FLT3 inhibitor to treat sizable segment of AML population - BTK inhibitor to treat B cell cancer patients resistant to / discontinuing Imbruvica - Exercised Exclusive License Agreement and Captured China Rights in 2018 - Expect to file IND for AML and CLL clinical trials in 2018 Announced Licensing Deal for Our Dual BET/Kinase Program Strong Leadership and KOL Support Strengthened Financial Foundation - Cash runway >12 months

Thank You!